Research Interests
Research Interests
T cells are key players in the immune system to fight against invading pathogens and cancer cells. T-cell based immunotherapies have been successfully applied in clinic to treat cancers and other diseases. However, current therapies show limited efficacy and adverse effects so that more mechanistic studies of T cell biology need to be performed to pave the way for next-generation immunotherapies.
In the past years, the Xu lab has developed cutting-edge biochemical and biophysical tools to study transmembrane signaling of T-cell receptor, co-stimulatory and co-inhibitory receptors. In addition, we also study lipid metabolism of T cells and have demonstrated the clinical potential of lipid-based immunotherapy.
Immunoreceptor signaling
A large number of receptors expressed on T cell surface orchestrate precisely to regulate adaptive immunity. These receptors can be classified to antigen receptor (TCR), co-stimulatory receptors (CD28 and others) and co-inhibitory receptors (PD-1 and others). Our group is interested in understanding the transmembrane signaling and protein regulation mechanisms of these receptors.
Immunoreceptors on T cell surface normally engage with membrane-anchored ligands and elicit ligand-dependent signaling through phosphorylation of tyrosine-based motifs. It is well known that tyrosine phosphorylation of immunoreceptors are regulated by kinases and phosphatases, but less is known about the role of neighboring lipid molecules. We find that acidic phospholipids can ionically interact with basic residue-rich sequence (BRS) in juxtamembrane regions of immunoreceptors to sequester tyrosine phosphorylation sites within the membrane bilayer and therefore avoid spontaneous activation. Such an ionic protein-lipid interaction is dynamic and can be influenced by local charge, mechanical force, membrane rigidity and curvature. We find that Ca2+ influx following T cell activation not only regulates downstream signaling but also modulates TCR and CD28 phosphorylation through the disruption of ionic protein-lipid interaction. Mechanistically, influxed Ca2+ ions transiently accumulate at channel pore region and directly interact with phosphate groups of acidic phospholipids to neutralize negative charges, which is specific and reversible. In comparison, other abundant cations in cytosol, such as Mg2+ and K+, show much weaker lipid binding due to their chemical properties. Importantly, this lipid/Ca2+ regulation is generally applicable to a diverse range of receptors like TCR, CD28 and LFA1 in T cells as well as IgG-BCR in B cells (Cell 2008, Nature 2013, Nat Comm 2015, Nat Rev Immunol 2016, Nat Struct Mol Bio 2017, PLOS Bio 2018), and the BRS is a common motif found in human single-pass transmembrane proteins, typically located in the cytoplasmic juxtamembrane region (Juxtamembrane sequences of human single-pass TM proteins table).
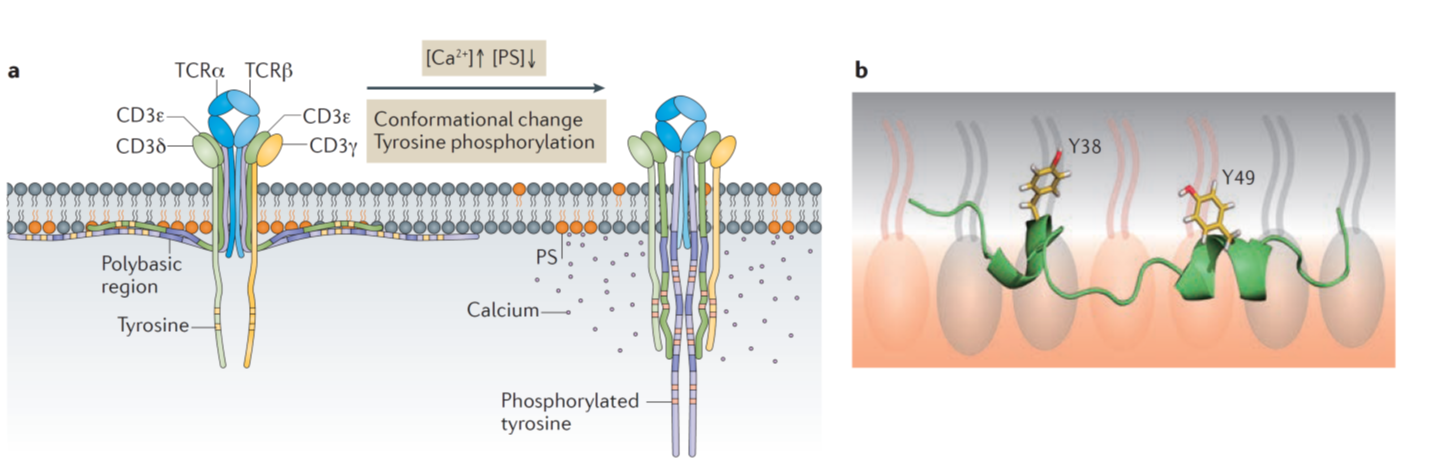
Reciprocal regulation of TCR signaling by acidic phospholipids and Ca2+.
(a) Acidic glycerophospholipids sequester immunoreceptor tyrosine-based activation motifs (ITAMs) of CD3 and CD3 cytoplasmic domains within the membrane bilayer, thus preventing the spontaneous T cell receptor phosphorylation and signaling in quiescent T cells. Upon antigen stimulation, multiple factors regulate the dissociation of ITAMs from the membrane to permit signaling. (b) Nuclear magnetic resonance structure of membrane-bound CD3 ITAM. The key tyrosine residues insert deeply into the membrane interior. PS, phosphatidylserine; TCR, T cell receptor. (Cell 2008, Nature 2013, Nat Rev Immunol 2016)
In addition to immunoreceptor phosphorylation process, another interest in the lab is to understand the basic biology of co-inhibitory receptors, in particular PD-1. Despite of its clinical importance, PD-1 signaling is still poorly understood. Key questions, for example, why PD-1 is abnormally upregulated in tumor microenvironment and how PD-1 suppresses T cell activation, remain to be addressed. We recently discover a degradation mechanism of PD-1 (Nature 2018). Surface PD-1 molecules are under constant internalization, and a E3 ligase FBXO38 mediates K48-linked polyubiquitination of internalized PD-1 that leads to proteasome degradation. This degradation mechanism is deficient in tumor infiltrating T cells because of low transcription level of Fbxo38 gene. Administration of IL2 is able to rescue FBXO38 expression to degrade PD-1 and reinvigorate T cell antitumor function. As one of the first-generation immunotherapy medicines, IL2 has already been approved to treat melanoma and kidney cancer. Thus, it should be of high interest to reconsider IL2’s application in different types of cancer. We will continue to investigate the signaling and regulation mechanisms of PD-1 under diverse disease contexts.
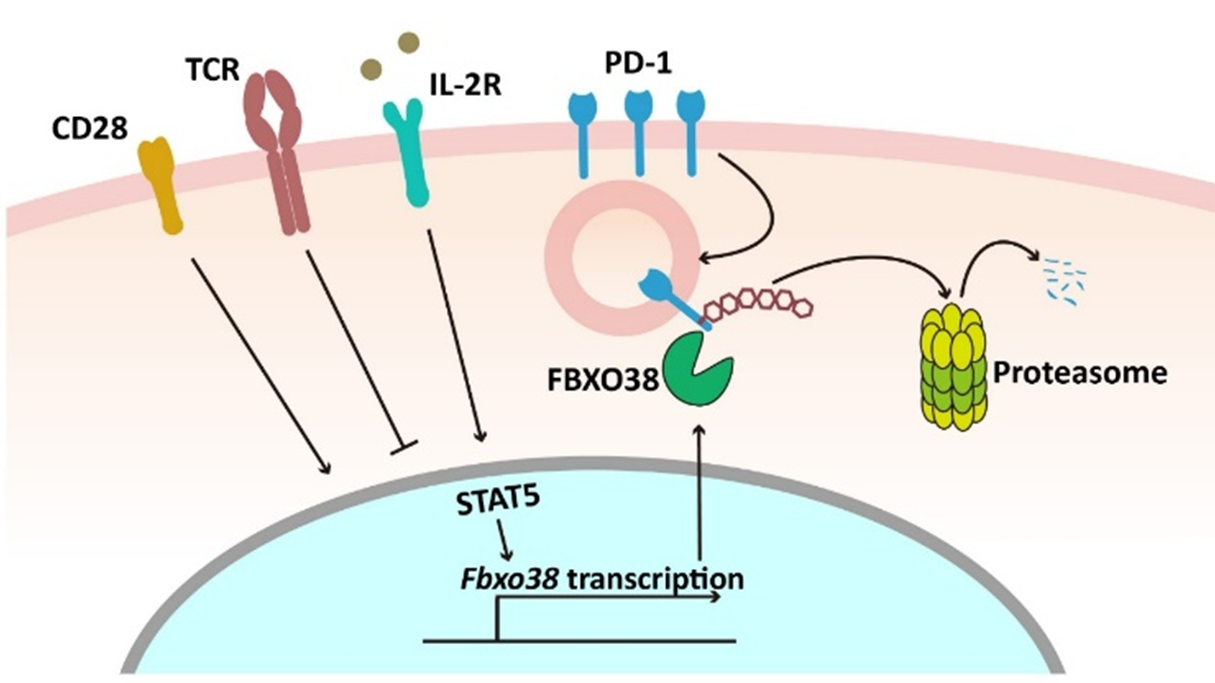
Degradation of PD-1 in T cells.
Surface PD-1 molecules can be internalized, ubiquitinated and degraded in proteasome. FBXO38 mediates K48-linked polyubiquitination of internalized PD-1. In tumor infiltrating T cells, Fbxo38 transcription is downregulated because of chronic antigen exposure and deficiency of CD28 and IL2 signaling. IL2 therapy can rescue FBXO38 expression in TIL through STAT5 activation, which in turn downregulates PD-1 surface level and reinvigorates T-cell antitumor activity. (Nature 2018)
Lipid metabolism
We recently start to explore the less-known immunometabolism field. Tumor bed is usually considered as a metabolic desert where T cells suffer from persistent nutrition deficiency stress. Moreover, some cancer-secreted metabolites were reported to exert diverse immune suppression functions. Hence, reprogramming T-cell metabolism would be necessary to improve cell survival and effector function against cancer cells. Moreover, metabolic regulation can be combined with signaling regulation to achieve better therapeutic effect. We found that inhibition of ACAT1, a key cholesterol esterification enzyme, augments TCR signaling and promotes immunological synapse maturation in CD8+ T cells. A pharmacological ACAT inhibitor (Avasimibe), which was previously used for cardiovascular diseases, potentiates the antitumor response of CD8+ T cells in preclinical models. A combined therapy of Avasimibe and anti-PD-1 shows better efficacy than monotherapies in controlling tumor progression, because the two reagents act through different mechanisms (Nature 2016). Following this work, we will keep exploring the complicated network of lipid metabolism and develop more lipid-based immunotherapies.

Metabolic regulation of antitumor immunity.
Metabolic stress in tumor microenvironment is one of the major causes of T-cell exhaustion. Inhibition of a cholesterol esterification enzyme ACAT1 reprograms cholesterol metabolism in T cells, leading to higher cholesterol level at the plasma membrane. Elevated cholesterol then contributes to better TCR clustering and immunological synapse formation. ACAT1 inhibition therefore promotes TCR signaling and T-cell killing function. An old drug (Avasimibe), targeting ACAT1 for cardiovascular disease, has been repurposed to treat cancer in our study. Avasimibe acts as a good antitumor reagent by itself or in combination with PD-1 blockade antibody. (Nature 2016)
Future directions
In the next five years, the Xu lab will mainly focus on the following subjects:
1. Lipid metabolic programs of T-cell subsets under different physiological and pathological conditions.
2. Functional roles of lipid metabolism in T-cell antitumor immunity.
3. Identification of T-cell “metabolic checkpoints” for cancer immunotherapy.
4. Immunoreceptor regulation and signaling